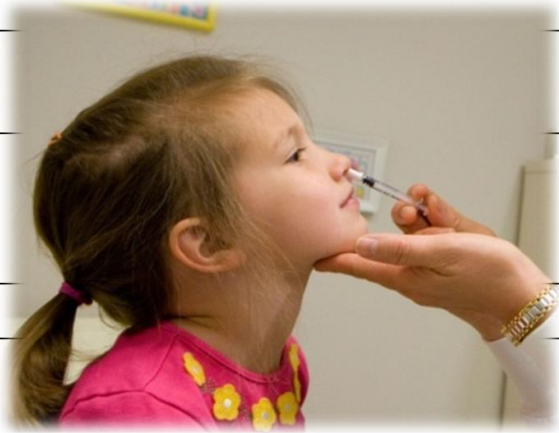
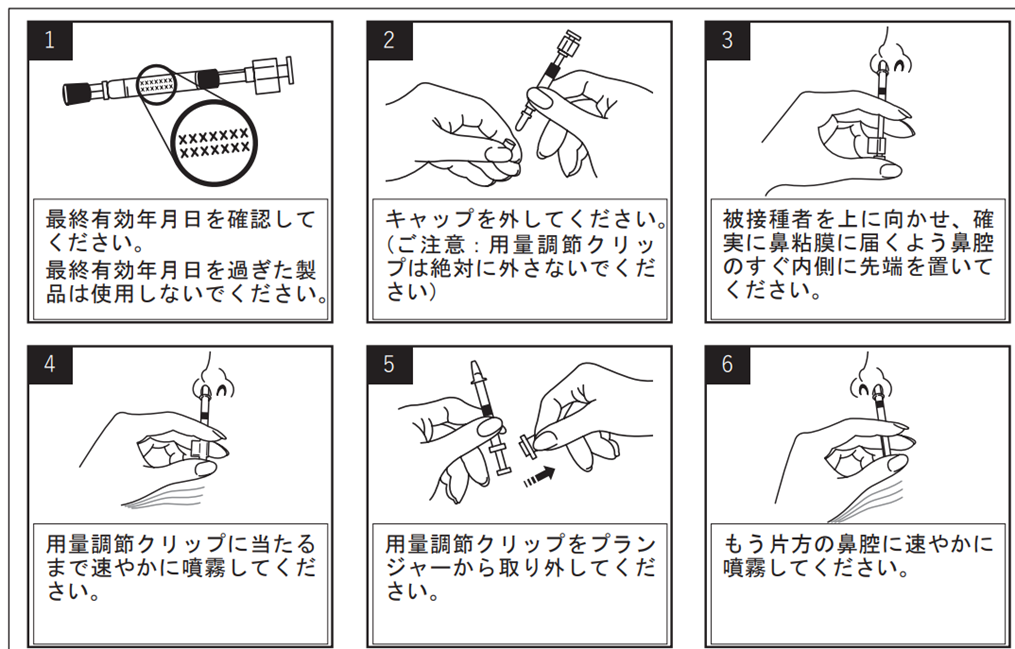
Appointment Start Date
Online appointments begin on Saturday, September 13, 2025 via our clinic website.WEB予約開始
📅 Vaccination Period
Scheduled from Wednesday, October 1 to Saturday, December 27, 2025
🟢 Eligibility
Children and adolescents aged 2 to under 19 years
💉 Administration Method
One dose of 0.2 ml sprayed into both nostrils (0.1 ml per side)
💰 Cost
¥8,000 (tax included)
Akita City subsidy of ¥1,000 applies → out-of-pocket cost: ¥7,000
📱 How to Book
Appointments available via our website. Reservations will close once the supply limit is reached.
📋Request to Complete the Pre-Visit Questionnaire
• To help reduce waiting time at the clinic,we kindly ask that you complete the pre-visit questionnaire (internal link) in advance,except for the “body temperature” field, which will be measured upon arrival. Please print the completed form and bring it with you on the day of your visit.
• 秋田市で10月1日にお子さんと妊婦さんのインフルエンザワクチン接種費の一部助成が決まりました。
👶 Eligible Recipients
At the time of vaccination, individuals must be registered residents of Akita City and meet one of the following criteria:
📅 Subsidy Period
October 1, 2025 (Wednesday) to February 28, 2026 (Saturday)
Please note: Our clinic will offer vaccinations from October 1 to December 27, 2025.
• 💡 Differences in ApplicationProcedure Based on Vaccination Timing
🔀For vaccinations received October 1–16, 2025 (Reimbursement method)
•
Please pay the full vaccination fee at the clinic first. Then, apply for the subsidy through Akita City’s online application system.
申請期限 令和8年3月31日
[Electronic Application Form for Influenza Vaccination Subsidy (External link)]
🔀10月17日以降に接種した場合市と契約した医療機関で接種(代理受領方式)
- As in previous years, submit the application form to the clinic in advance.
→ 当院が助成の手続きを代わりに行います。その後の手続きは不要です。
Please fill out the proxy authorization form using a non-erasable pen. Applications with corrected amounts cannot be accepted. If you make a mistake, please fill out a new form.
• 委任状は以下のものか当院に備え付けのものをご使用ください。
Influenza Vaccination Subsidy Application & Proxy Authorization Form (Excel, 17.7 KB)
This is the application and proxy authorization form. Please fill in the highlighted sections. The form is also available at our clinic.
Influenza Vaccination Subsidy Application & Proxy Authorization Form (PDF, 36.5 KB)
This is the handwritten version of the application and proxy authorization form. It is also available at our clinic.
Sample Completed Form (PDF, 46.9 KB)
This is a sample of how to fill out the application and proxy authorization form.
📝 申請書の事前記入のお願い 10月1日以降、当院窓口に申請書を置いてありますが
To reduce congestion in the clinic, please complete the form before your visit and bring it with you on the day of vaccination.
💴 Subsidy Amount and Dosing
• 助成額:1回あたり1,000円
• 生後6か月〜13歳未満のお子さんは2回接種で合計2,000円の助成
Pregnant women are eligible for one subsidized dose
🏥 For Residents Outside Akita City
• People living outside Akita City can also receive the influenza vaccine at our clinic.
• 接種費用の補助は各市町村に申請して受けられますので、お気軽にお問い合わせください。
There are two main types of influenza vaccines. Both the World Health Organization (WHO) and the National Institutes of Health (NIH) recommend selecting the appropriate vaccine based on the patient’s age, health condition, and living environment.
| 比較項目 | LAIV(フルミスト®) | IIV(不活化ワクチン) |
| 接種方法 | 鼻腔噴霧(痛みなし) | 皮下注射(痛みあり) |
| 対象年齢 | 2~18歳(日本) | 生後6か月以降 |
| 有効性 | 同等 | |
| 粘膜免疫(IgA)を誘導し、感染初期の防御に優れる。 | 血中抗体(IgG)を誘導し、重症化予防に優れる。 | |
| 副反応 | 鼻水・咳など軽度 | 腫れ・発熱など |
| 接種制限 | 妊婦・免疫不全者は不可 | 幅広く接種可能 |
• In our experience, FluMist® may be a suitable option for children who have previously experienced significant swelling from injectable influenza vaccines, such as reactions extending beyond the elbow. Please consult with our staff to determine the best choice for your child.
🔗 Related Internal Links
🏙️ Announcement: 2025 Influenza Vaccination Appointments Now Open
- 🏙️ [Akita City Influenza Vaccination Subsidy Information]
🧓Senior Influenza Vaccination Guide – 2025
🛡️Frequently Asked Questions: Inactivated Influenza Vaccine vs. Nasal Spray (FluMist®)
References and Source MaterialsClick to view the recommended documents and guidelines.
• “Both LAIV and IIV are effective when matched to circulating strains. Selection should be based on age, health status, and epidemiological context.”
(訳:LAIVもIIVも流行株に合っていれば有効。選択は年齢・健康状態・流行状況に応じて行うべき)
WHO FluNet Surveillance Dashboard
• FluMist. FDA Drug Label.
米国食品医薬品局(FDA)DailyMed
• Thwaites RS, Uruchurtu ASS, Negri VA, et al.
Early mucosal events promote distinct mucosal and systemic antibody responses to live attenuated influenza vaccine - PubMed
Nature Communications. 2023;14(1):8053. doi:10.1038/s41467-023-43842-7
• Barría MI, Garrido JL, Stein C, et al.
Localized Mucosal Response to Intranasal Live Attenuated Influenza Vaccine in Adults.
The Journal of Infectious Diseases. 2013;207(1):115–124. doi:10.1093/infdis/jis64
🌍 WHO(世界保健機関)の見解(2024年版)
🇺🇸 NIH(米国国立衛生研究所)およびACIPの見解(2025年春季報告)“LAIV is a safe and effective option for healthy individuals aged 2–49, but IIV remains the preferred choice for immunocompromised and pregnant populations.”
(訳:LAIVは健康な2〜49歳にとって安全で有効な選択肢だが、免疫不全者や妊婦にはIIVが推奨される)
• CDC: Trivalent Influenza Vaccines
• 2024–2025 Flu Season
🧑⚕️ 日本小児科学会の見解(2024年9月2日付)
• 2024/25シーズンに向けたインフルエンザワクチン接種に関する考え方とトピックス
• 経鼻弱毒生インフルエンザワクチンの 使用に関する考え方(日本小児科学会 外部リンク)
• 小児に対するインフルエンザワクチンについて(厚労省 外部リンク)
• フルミスト点鼻液 添付文書 (第一三共資料 外部リンク)